PAEDIATRICS & NEURO-DEVELOPMENT
MedTech for One Healthy Baby: How can Technology support the ‘Halveit Campaign” to reduce stillbirth and neonatal brain injury?
The NIHR Brain Injury MIC in collaboration with NHS Improvement and NHS England developed a three-stage process to identify an area where technology could be developed and utilised to improve the care of mothers and their babies.
The Brain Injury MIC has organised a series of workshops designed to provide technology-based solutions to support the government campaign to reduce stillbirths in the UK. The first workshop – led by Professor Topun Austin – took place in London in September 2018 and was attended by experts from all over the country from Universities, the National Institute of Health Research (NIHR), infrastructures and by a parent representative.
This facilitated workshop, informed by the ecosystem map enabled key areas to be interrogated and key topics to emerge. Through this process the following three themes emerged:
- Data linkage from conception to delivery: risk assessment & data integration
- Smart fetal assessment
- Service Delivery
Having developed 3 exploratory pathways we now wish to optimise these outputs to develop a final workstream that could potentially attract funding to assist the national ambition to reduce stillbirths and neonatal brain injury. This will be achieved through a collaboration with the Engineering Design Centre in Cambridge, who specialise in systems engineering approaches to healthcare design.
More info:

Research Strategy
The strategy of our Paediatric and Neurodevelopmental theme is complimentary to the Paediatric theme of the Cambridge BRC which is building a collaborative group of clinical, technology- and laboratory-based investigators to provide expertise in basic and translational research relevant to Paediatrics through Next generation sequencing (NGS), bioinformatics and deep phenotyping. A perinatal neuroscience group is being developed in Cambridge to identify/stratify neonates with brain injury (HIE, stroke and white matter damage) and neurological rare disease, building on the CUH Neuro-NICU that cared for 229 (23% of all NICU admissions) patients in 2015. Early detection is key to neuroprotective approaches.
Aims and specific short (1 year), medium (2-3 years) and long (4-5 years) objectives
Publication and dissemination of road mapping workshops in Paediatric Neurorehabilitation and Neonatal Neurocritical Care orchestrated by the current HTC in 2016:
- Continue to collaborate with the TITCH network
- Establish access for patients and families to record their experiences on the ORION platform
- Catalyse further development to bedside implementation:
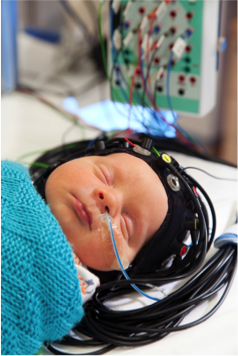
- Neonatal neuromonitoring (Austin/Smielewski): novel non-invasive indicators of cerebro vascular reactivity.
- Neonatal neuroimaging and neurovascular coupling using Diffuse Optical Imaging and development of MR functional brain imaging (Austin in collaboration with UCL–neoLAB, Baron Cohen, Autism Research Centre, Suckling, Psychiatry).
- Multimodality Monitoring in Paediatric Neuro-intensive Care (Young/Hutchinson/Czosnyka/Smielewski)
- Study of Novel Proteomic Biomarkers of Brain Injury in Term Newborns with Hypoxia-Ischemia (Divyen Shah).
- Seizures: remote detection of seizures initially in adults with cognitive impairment; extra cranial detection of seizures during sleep (Noctusense)
New subthemes to develop platforms and technologies:
- Develop a national perinatal neuroscience research group to identify and investigate neonates with brain injury, neurological rare diseases and atypical neurocognitive phenotypes
- Early Detection and Stratified Medicine for Neonatal Seizures
- Implementation of Diffuse Optical Imaging to diagnose neonatal (and eventually) adult large vessel stroke
- Identify hypothermia-induced neuroprotective pathways (eg, RMB3) human neonates with hypoxic-ischemicencephalopathy (HIE)
- Improve clinical outcomes in children through (i) personalised care using novel predictive algorithms, and (ii) individualized interventions (e.g., anti-epileptic drug repurposing); both based on enhanced mechanistic understanding of pathways leading to adverse and atypical outcome.
- Training component: to train the next generation of investigators and leaders with training posts in Years 3-5.
Subthemes
The prevalence of cerebral palsy is rising in the UK because of increased survival of extremely low birth weight (ELBW) preterm infants. About 10% of all babies delivered in the UK spend time in special care neonatal units, and the annual number of recorded days of care is >1 million days (ONS 2014). Neonatal seizures are the most common neurological emergency and are associated with high mortality and morbidity and thus even small improvements in care have the potential to be highly significant over the life course. The incidence of neonatal seizures in term infants is 0.5-3 per 1000 live births, and most epidemiological studies only report clinical seizures but rarely sub-clinical seizure states. Thus, a conservative prediction is that there are about 2,000 cases per annum (PA) in the UK.
The primary objectives of this Consortium scheme, ‘The NeuralNET,’ are to develop systems for Early Detection and Stratified Medicine for neonatal seizures as the basis for novel methodological, technical and interventional research and to create a National Platform to support outreach, patient and industry engagement, promote clinical trials and training of young investigators.
Our objectives are:
- To demonstrate that stratification of neonatal seizures using magnetic resonance imaging (MRI), EEG and whole genome sequencing (WGS) is feasible (industry collaboration with Illumina UK, Cambridge,UK);
- To develop, with Illumina, a rapid turn around pipeline for WGS and informatics taking a minimal (1ml) blood volume from babies with report back of “actionable” information to clinicians within 2 weeks;
- To use an inter-regional approach to identify large cohorts of stratified patients in the NICU, creating a unique opportunity to study interventions in an early, potentially enhanced therapeutic window;
- To create a new paradigm for stratified medicine in paediatrics coupled to assessment of long-term outcomes using the electronic medical record, which captures a unique and inherent strength of the NHS organization for population research studies (industry collaboration with Clevermed, Edinburgh,UK).
- To enable targeted clinical trials and encourage industry input through open innovation, the provision of stratified cohorts and data, and through provision of a feasible stratification toolkit;
- To support planning for a clinical trial for Glut1syndrome (industry link with Ultragenyx, Novato, CA, USA);
- To develop clinically and test machine learning tools for MRI and EEG interpretation to improve clinician and cotside recognition of brain injury and sub-clinical seizures for treatment;
- To prospectively identify and treat NICU patients with KCNQ2 encephalopathy (~100cases/PA in the UK) with the repurposing drug carbamazepine to control seizures and assess long term outcomes of this cohort using the electronic medical record to determine the effects of early treatment:
- NeuralNET as a National Platform:
- To provide a novel platform for industry-sponsoredclinical trials (which will otherwise not proceed)without a coordinated national approach;
- To prospectively establish incidence of genetic disordersfor trials of diagnostics and rare disease treatments (industrylinks: Roche, Basel, SZ and Healx, Cambridge, UK);
- To create a national bioresource and database of cases for future studies and encourage future contribution to and use of this valuable resource for mechanistic studies (link: NIHR Rare Disease BioResource);
- In consultation with NICE, consortium findings and cost-effectiveness studies would directly influence care pathway management recommendat ions in the UK.
The NeuralNET will recruit 600 babies from England and Scotland; we expect to stratify the 600 subjects into the following categories; (i) Injury (incl. HIE, Stroke, Infection)=400; (ii) Rare including KCNQ2 benign and severe, KCNT1, SCN2A and other genetic=80; (iii) Metabolic (incl. hypoglycaemia)=60-including transient metabolic and in born errors of metabolism; (iv) Malformations=60.
These numbers are estimates based on historical data.
Neonatal stroke is a significant cause of lifelong neurodisability and epilepsy. There is currently no reliable way of diagnosing stroke early on (eg, at the cotside) nor any treatment to reduce or minimise brain injury. Neonatal stroke can present with seizures in the first few days of life, although altered consciousness or tone may be the only signs. Diagnosis is made by imaging the brain. Ultrasound of the brain is simple and portable, however is not very good at detecting stroke. The ‘gold standard’ imaging technique is magnetic resonance imaging (MRI) but access to these scanners can be difficult and the average time for MRI after presentation is 3 days.
Diffuse optical imaging (DOI) is a safe non-invasive way of measuring perfusion and brain oxygenation at the cotside. DOI measurers near-infrared light (just beyond the visible) across the brain and changes in light absorption are seen with changes in blood flow and oxygen levels. Using multiple light sources and detectors it is possible to obtain images of blood flow and oxygen levels in different parts of the brain.
We aim to:
- Modify existing technology to develop a portable DOI system for cotside assessment.
- Validate the system in term infants at risk of neonatal stroke against MRI.
Dr Rob Cooper (UCL) is holder of an EPSRC early career fellowship to develop wearable neuroimaging technologies for the neonatal intensive care unit (EP/N025946/1) in collaboration with start up industrial partner GowerLabs (www.gowerlabs.co.uk/).
Neonatal hypoxic-ischemic encephalopathy (HIE) affects 1-3/1000 live births in the US leading to long-term neurological sequelae including learning difficulties, cerebral palsy or even death.Therapeutic hypothermia (33.5oC for 72 hours) is an effective treatment for neonatal HIE and is offered as a standard of care. However, the cellular and molecular mechanisms underlying the beneficial effects of therapeutic hypothermia are poorly understood. Our hypothesis is that therapeutic hypothermia induces the “hibernome”, comprising genes that are neuroprotective for HIE. Giovanna Mallucci’s lab in Cambridge recently showed that hypothermia-induced RBM3 promotes synaptic regeneration in adult neurodegenerative mouse models and is involved in synaptic maintenance of wild type mice (Peretti et al, Nature 2015) . Our unpublished data shows that cooling induces RBM3 in humans also, which is detectable in blood.
In collaboration with Divyen Shah, QMH NICU), we will:
- establish an ELISA assay system to screen for RBM3 expression the serum of human neonates undergoing hypothermia
- establish the time course of RBM3 expression at three points during the 72-hr hypothermia protocol and investigate links of RBM3 expression pattern to neonatal neurological outcomes